EVIDENCE OF EVOLUTION
Radiometric and Astronomical Dating
[Credit]
Chapter Outline
- Introduction
- Radiometric and Astronomical Dating
- Fossils
- Biogeography
- Homologous Structures
- Vestigial Organs
- Developmental Similarities
- Molecular Evidence
Links to external sites will appear in pop-up windows.
Radiometric Dating
To determine the age of an igneous rock, the rock is crushed and analyzed by a mass spectrometer. The mass spectrometer can tell which elements are in a rock. Uranium is an element that goes through radioactive decay and becomes lead. Every 4.5 billion years, half of the Uranium-238 in a rock will decay into Lead-206. This means that U-238 has a half-life of 4.5 billion years. This rate of decay is constant and predictable, so we can use it to determine the age of a rock. If a rock has 4 times as much uranium as it does lead, that means less than one half-life has passed, and the rock is approximately 1.7 billion years old (see figure 1).
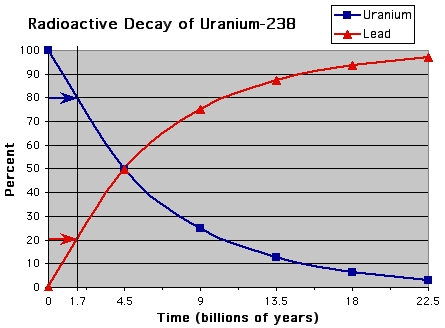 |
| Figure 1 - Click to enlarge |
Potassium-40 is also used to date rocks. Potassium is a component of the minerals called potassium feldspar (containing potassium, aluminum, slicon, and oxygen), biotite (potassium, magnesium or iron, silicon, aluminum, oxygen, and hydrogen) and muscovite (potassium, aluminum, silicon, and oxygen) among others. Granite is a common rock made up of potassium feldspar, biotite, and muscovite along with quartz (made of silicon and oxygen), plagioclase (sodium or calcium, silicon or aluminum, and oxygen), and amphibole (some combination of calcium, magnesium, iron, sodium, and aluminum attached to silicon, oxygen, and hydrogen) . As you can see, there is no argon, the decay product of Potassium-40, in any of the minerals making up granite. However, as soon as a piece of granite forms, the Potassium-40 starts to decay into Argon-40, and the argon will be trapped in the rock. The half-life of P-40 is 1.3 billion years. If a piece of granite has the same amount of potassium and argon, that means a full half-life has passed, and the rock is 1.3 billion years old. If the rock has 7 times as much argon as it does potassium, that means three half-lives have passed, and the rock is 3.9 billion years old.
Resources:
- Geologic Timeline
- Geologic Time - William L. Newman, USGS
- Geologic Timescale
| [ Previous Page ] | [ Next Page ] |